EMBO Rep | 毛志勇团队发现转录因子PAX5激活人类LINE1逆转录转座子诱导细胞衰老
当细胞进入不可逆的周期阻滞状态时,称之为细胞衰老。细胞衰老与机体衰老、衰老相关疾病和肿瘤的发生密切相关。衰老细胞失去原有功能。这一过程伴随着染色质的重组,特别是包含占人类基因组17%的长散在核元件1(LINE1)的异染色质区域变得更松散和更易接近,使得LINE1解除抑制
虽然已发现许多转录因子在不同生物学背景下调控LINE1转录,但在衰老细胞中,FOXA1是唯一已知的结合LINE1并增强LINE1转录的因子
近日,3344体育官方入口毛志勇教授团队在EMBO reports发表题为The transcription factor PAX5 activates human LINE1 retrotransposons to induce cellular senescence的研究成果,该研究发现调控人LINE1逆转录转座子的新转录因子PAX5。PAX5在5'-UTR的富集激活LINE1并促进细胞衰老和SASP的分泌。该团队亦发现,长寿基因SIRT6结合于PAX5启动子并抑制PAX5表达以抑制LINE1转录,从而抑制细胞衰老。

在该项工作中,研究团队通过分析不同类型衰老细胞中共同上调的LINE1元件的5'非翻译区中的保守结合基序,预测了几种与人类LINE1元件结合以调节其转录的转录因子,并结合LINE1 5'-UTR与荧光素酶融合报告载体筛选并鉴定出PAX5为LINE1启动子活性的新正调控因子。在衰老细胞中,观察到PAX5表达增加,并且PAX5在LINE1元件5'-UTR区域的结合增强,从而促进LINE1转录,加强细胞衰老并促进SASP分泌。研究团队还证明了长寿基因SIRT6通过直接结合PAX5启动子抑制PAX5的转录,而PAX5的过表达消除了SIRT6对压力依赖性细胞衰老的抑制作用。
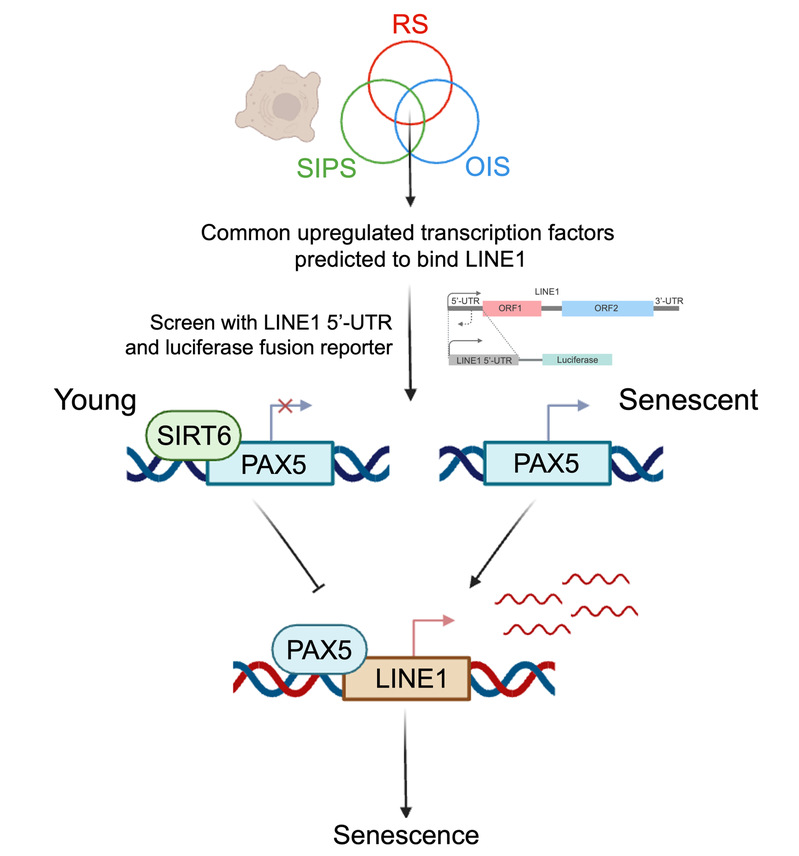
图 筛选LINE1调控转录因子及PAX5调控LINE1促进细胞衰老模式图
综上所述,该研究团队建立了一种基于细胞衰老中共同上调的LINE1转录本,识别并筛选富集转录因子的方法。阐述了PAX5调控LINE1并促进细胞衰老的分子机制,以及长寿基因SIRT6对PAX5调控的分子机制。该研究发现调控LINE1转录活性的新转录因子PAX5,拓宽了细胞衰老进程中LINE1的调控机制,为抑制LINE1激活和治疗衰老相关疾病的药物开发,提供了全新的靶点。
3344体育官方入口附属妇产科医院/3344体育官方入口博士后唐欢胤和博士研究生杨佳庆为本论文的共同第一作者。3344体育官方入口附属妇产科医院/3344体育官方入口毛志勇教授为本文的通讯作者。毛志勇教授课题组的许君豪博士研究生、耿安珂助理教授、张伟娜助理教授和蒋颖副教授也参与了该项工作。
参考文献
[1] Treangen TJ, Salzberg SL. Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nature Reviews Genetics, 2012, Vol.13(1): 36–46.
[2] Cecco MD, Ito T, Petrashen AP, et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature, 2019, Vol.566(7742): 73–8.
[3] Thomas CA, Tejwani L, Trujillo CA, et al. Modeling of TREX1-Dependent Autoimmune Disease using Human Stem Cells Highlights L1 Accumulation as a Source of Neuroinflammation. Cell Stem Cell, 2017, Vol.21(3): 319-331.e8.
[4] Simon M, Meter MV, Ablaeva J, et al. LINE1 Derepression in Aged Wild-Type and SIRT6-Deficient Mice Drives Inflammation. Cell Metabolism, 2019, Vol.29(4): 871-885.e5.
[5] Tasdemir N, Lowe SW. Senescent cells spread the word: non‐cell autonomous propagation of cellular senescence. The EMBO Journal, 2013, Vol.32(14): 1975–6.
[6] Campisi J. Aging, Cellular Senescence, and Cancer. Annual Review of Physiology, 2013, Vol.75(1): 685–705.
[7] Acosta JC, Banito A, Wuestefeld T, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature cell biology, 2013, Vol.15(8): 978–90.
[8] Hoare M, Ito Y, Kang T-W, et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nature cell biology, 2016, Vol.18(9): 979–92.
[9] Sun X, Wang X, Tang Z, et al. Transcription factor profiling reveals molecular choreography and key regulators of human retrotransposon expression. Proceedings of the National Academy of Sciences of the United States of America, 2018, Vol.115(24): E5526–35.
[10] Tang H, Geng A, Zhang T, et al. Single senescent cell sequencing reveals heterogeneity in senescent cells induced by telomere erosion. Protein & Cell, 2019, Vol.10(5): 370–5.
[11] Teo YV, Rattanavirotkul N, Olova N, et al. Notch Signaling Mediates Secondary Senescence. Cell Reports, 2019, Vol.27(4): 997-1007.e5.
[12] Tao W, Yu Z, Han J-DJ. Single-cell senescence identification reveals senescence heterogeneity, trajectory, and modulators. Cell Metabolism, 2024.
Copyright© 2011-2015 3344体育官方入口 - 3344体育网平台
地址:上海市四平路1239号 电话:021-65981041 传真:65981041


